Laboratory commercial feed tests provide information on protein, level and digestibility of fiber, minerals, which are all factors that determine the feeding quality of the forage. Today many forage tests provide information beyond the energy and protein in the feed but also feed fermentation quality and its stability in the manger. Having efficient fermentation is critical to ensure the forages being fed are highly palatable and digestible.
There are a few lab results that tell us how well the feed is fermented which include:
- Moisture/ Dry Matter (DM).
- Major fermentation acids produced: lactic, acetic, propionic, and butyric.
- Protein fraction converted to ammonia (Ammonia-CP) during fermentation.
- Protein fraction bound to fiber via excessive heating during ensiling is Acid Detergent Insoluble Crude Protein (AD-ICP).
- Remaining ethanol or water-soluble sugar (ESC or WSC) fractions are not converted to acids during fermentation.
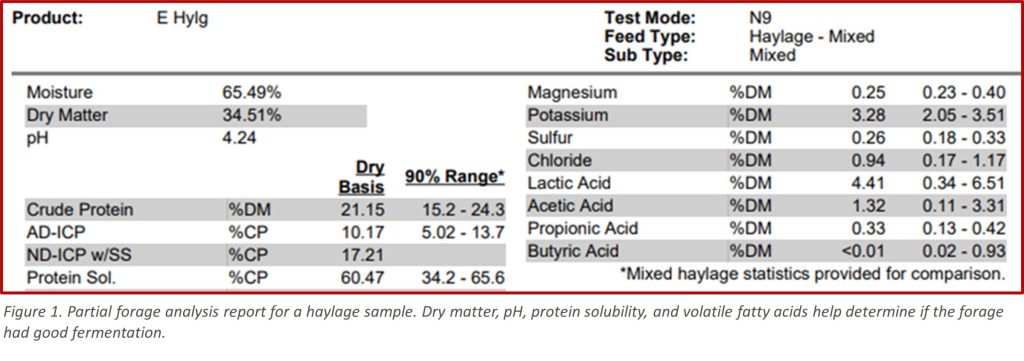
DM/Moisture: Desirable fermentation is most likely in the range of 35-45% DM or 55-65% moisture, depending on the forage and storage structure used. Both haylages that are too wet or too dry may have poor fermentation. Dry haylage will be harder to exclude oxygen out of the silo/pile. Oxygen must be depleted to begin anaerobic fermentation. Wet forage will encourage a clostridial fermentation which is not desirable. A slower fermentation will deplete desirable sugars and produce excess heat. The sample haylage in Figure 1 is wetter than average, but still within the range where good fermentation can be achieved.
pH: Is key for evaluating the fermentation process. For the most part, when pH is within the normal range, the lower the pH the better the fermentation. This pH is 4.24 in the range of low enough to make the feed stable.
Lactic, Acetic, Propionic, and Butyric Acids:
Terminology
- acetic acid = acetate
- lactic acid = lactate
- propionic acid = propionate
- butyric acid = butyrate
Lactic acid is the predominant fermentation acid found in silages. Adequate levels of lactic acid indicate minimal dry matter losses and proper fermentation. Lactic acid production is a more efficient fermentation, losing less energy through fermentation than acetic acid production. The pH drops more rapidly with high lactic acid fermentation. Low lactic acid production can result from: restricted fermentation due to high DM content or cold weather, samples taken after considerable aerobic exposure, and silages affected by clostridial fermentation (high in butyric acid).
Acetic acid provides forage with a vinegar odor and taste but helps with aerobic stability by inhibiting the proliferation of yeasts and molds. High levels can be caused by extremely wet silage, prolonged fermentation, loose packing, or slow silo filling.
Propionic acid provides forage with a sweet smell and taste. Very low levels of this acid are found in well fermented forages.
Butyric acid (butyrate) is often associated with wet forages and increases over time in storage, and it is caused by clostridial fermentation. What are the negatives associated with butyric acid in the forage? Butyric smells rotten, it is objectionable to the cow and intake will drop. Also, for transition cows that are prone to risk of ketosis, butyrate is a ketone, high butyrate forages are therefore already elevated for ketones, even when the liver will produce more, pushing the cow to ketone overload more easily. The prolonged butyric fermentation will deplete digestible energy sources such as sugars and lactic acid, lowering the digestibility of the feed, and causing the production of amines and ammonia.
| Typical concentrations of fermentation end products in legume, grass, & corn silages, and high moisture corn. | |||||
| Legume Silage (30 – 40% DM) | Legume Silage (45 – 55% DM) | Grass Silage (30 – 35% DM) | Corn Silage (30 – 40% DM) | High Moisture Corn (70 – 75% DM) | |
| pH | 4.3 – 4.7 | 4.7 – 5.0 | 4.3 – 4.7 | 3.7 – 4.2 | 4.0 – 4.5 |
| Lactic Acid (%) | 7 – 8 | 2 – 4 | 6 – 10 | 4 – 7 | 0.5 – 2.0 |
| Acetic Acid (%) | 2 – 3 | 0.5 – 2.0 | 1 – 3 | 1 – 3 | < 0.5 |
| Propionic Acid (%) | < 0.5 | < 0.1 | < 0.1 | < 0.1 | < 0.1 |
| Butyric Acid (%) | < 0.5 | 0 | 0.5 – 1.0 | 0 | 0 |
| Ethanol (%) | 0.2 – 1.0 | 0.5 | 0.5 – 1.0 | 1 – 3 | 0.2 – 2.0 |
| Ammonia-N (% CP) | 10 – 15 | < 12 | 8 – 12 | 5 – 7 | < 10 |
| DM = Dry Matter | CP = Crude Protein Adapted from: Kung and Muck. 2017. Silage Harvesting and Storage. Large Dairy Herd Management. | |||||
Most inoculants for haylage are primarily lactic acid producers, you can often tell an inoculated forage vs. one not inoculated by the amounts of lactic and acetic acid. Inoculated forages will be higher in lactic acid. This haylage has favorable levels of both lactic and acetic acids within the normal range found in haylage samples. Butyric acid is undesirable. Wet forages are best fed quickly before the VFA profile deteriorates.
Alfalfa is harder to ferment (drop the pH as low as quickly) than grass or corn silage. This is because alfalfa has high CP concentration and consequently elevated ammonia during fermentation. Ammonia buffers the pH from acid production.
Ammonia-CP: A high concentration of ammonia in haylages or grass silage indicates excessive breakdown of protein caused by slow drop of pH or clostridial fermentation. Depending on the total diet, wet haylage may provide excessive rapidly available protein compared to dryer haylage with a more desirable fermentation.
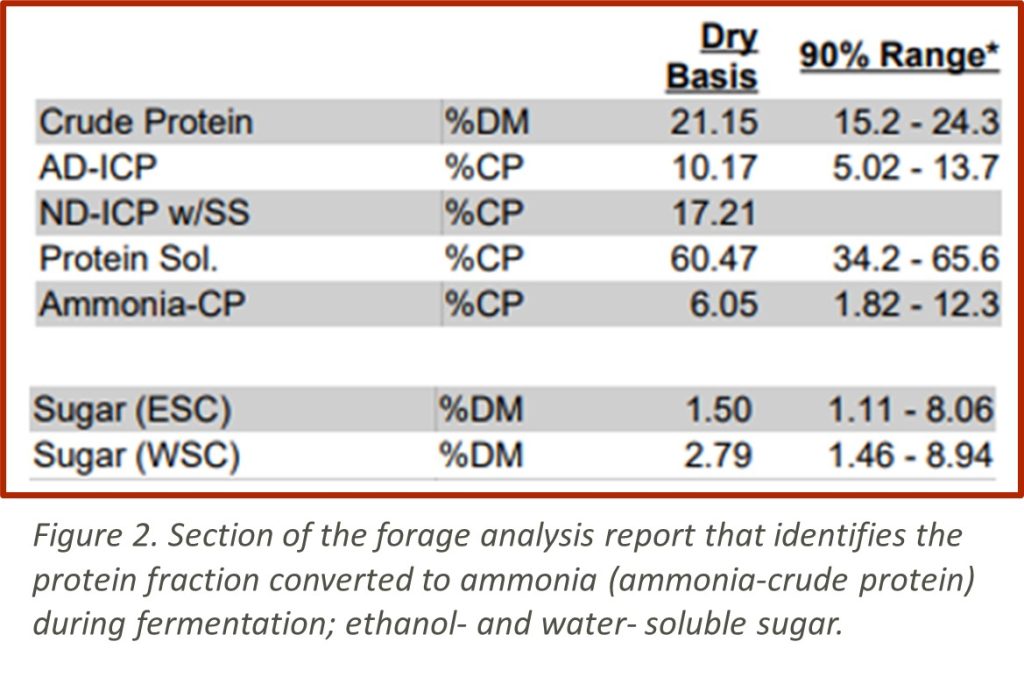
AD-ICP: If a forage heats too much in storage, some protein will become unavailable for digestion.
ESC or WSC Sugar: Higher sugar levels indicates these sugars will be available to feed heat producing bacteria when the silage is exposed to air at feed out.
Corn silage pH, fermentation acid profile, sugar, etc. have the same implications as for haylage. Here are some differences between corn silage and haylage:
- As direct harvested it is “cleaner” with less field “dirt” incorporated into the feed. The test that shows this is the ash level of the feed. The soil in haylage can be a source of clostridia spores and induce butyric acid producing fermentation.
- Corn silage is lower in minerals and ferments to a lower pH with less buffering capacity.
- Corn silage ferments rapidly and is high in sugar. There may still be abundant sugar after complete fermentation that will be available when the feed is exposed to oxygen at feed out. This is a potential stability problem with corn silage.
- An additional test to measure yeasts and molds may be a helpful indicator to measure feed stability.
- If inoculant including L. buchneri is used, elevated levels of acetate will develop over time. While acetic acid production in haylage is an indicator of slower, less energy-efficient fermentation, acetate is a superior acid for stability, providing resistance against secondary fermentation at feed out. L. buchneri slowly produces acetate while in the storage phase and acetate will increase over time. This is desirable because of the higher sugars often found in corn silage that can make a feed unstable at feed out.
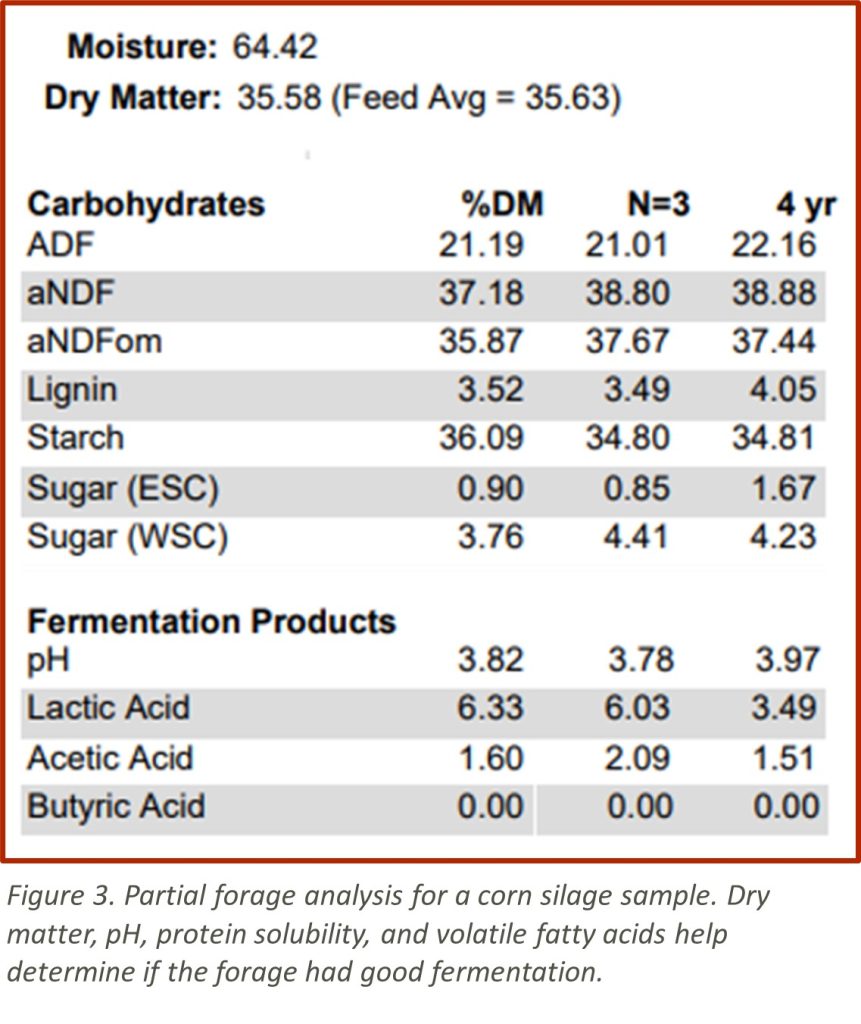
The examples provided (Figures 2 and 3) come from two different widely used Midwestern laboratories. Be careful comparing results across labs. These labs do provide information on how the sample compares to lab averages or ranges.
Good forage must be more than simply high in energy and digestibility, it must also be well-preserved and palatable to support high production in dairy cattle.
Resources
Amaral-Phillips, D. M. Fermentation analysis of silages. Kentucky Cooperative Extension Service, Lexington, KY. Accessed from: https://afs.ca.uky.edu/files/fermentation_analysis_of_silages.pdf.
Barnhart, Stephen and Sherry Hoyer. 2010. Interpreting your forage test report. Iowa State University Extension. Accessed from: https://www.iowabeefcenter.org/information/IBC51.pdf.
Kung, L. and R. Shaver. 2001. Interpretation and use of silage fermentation analysis reports. University of Wisconsin-Madison Extension. Accessed from: https://fyi.extension.wisc.edu/forage/interpretation-and-use-of-silage-fermentation-analysis-reports/.
Muck, R. E. 2006. Butyric acid in silage: why it happens. US Dairy Resource Center. Accessed from: https://www.ars.usda.gov/ARSUserFiles/50901500/dairyexpo/2006/WDE2006_butyric_acid.pdf.
Schroeder, J. W. 2004. AS-1254 Silage fermentation and preservation. NDSU Extension Service, Fargo, ND. Accessed from: https://library.ndsu.edu/ir/handle/10365/5102.
Reviewers:
Luiz Ferraretto | Assistant Professor & Extension Specialist of Ruminant Nutrition | UW-Madison
John Goeser | Adjunct Assistant Professor | UW-Madison





